Second Test for the BD COR™ MX to be CE Marked to IVD Directive
FRANKLIN LAKES, N.J., May 26, 2022 / B3C newswire / -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced a new high-throughput molecular diagnostic combination test for SARS-CoV-2 and Influenza A/B, the second test available for the BD COR™ PX/MX System to have been CE marked to the IVD directive 98/79/EC.
The BD SARS-CoV-2/Flu assay for the BD COR™ System is an automated multiplexed real-time RT-PCR test to detect and differentiate SARS-CoV-2 and influenza A, and/or influenza B from a single nasal sample from patients who are showing signs of respiratory viral infection, as well as those who are asymptomatic.
“As long as the COVID-19 pandemic persists or evolves to an endemic situation, testing for the virus will remain an important public health tool,” said Celine Roger-Dalbert, vice president diagnostic assays R&D at BD. “And while last year’s flu season was relatively tame, with many areas loosening guidelines and mask guidance, this season could see a resurgence in seasonal illnesses. It is important to quickly diagnose if a patient has COVID-19 or flu, and type of flu, to inform patient management and to treat appropriately and early in the course of the disease.”
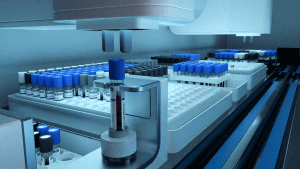
The BD COR™ MX/PX System allows 1,700 specimens to be loaded, with onboard capacity for reagents and samples that provide more than six hours of unimpeded system processing and up to 1,000 sample results in 24 hours, eliminating multiple technologist interactions currently required per shift. The BD COR™ MX instrument launched in 2021 with the BD CTGCTV2 molecular assay, a single test that detects the three most prevalent non-viral sexually transmitted infections (STIs) — Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC) and Trichomonas vaginalis (TV).
“The BD SARS-CoV-2/Flu assay is the first delivery on our promise to continue to expand assays that can be performed in high-throughput central testing labs,” said Brooke Story, president of Integrated Diagnostic Solutions for BD. “We will continue to broaden our available tests for the BD COR™ System, including assays for other common STIs, overall women’s health and gastrointestinal infections.”
The BD COR™ System integrates and automates the complete molecular laboratory workflow from sample processing to diagnostic test result. The BD COR™ PX instrument focuses on sample workflow for diagnostic specimens and assays, preparing the samples by performing the appropriate pre-analytical processing steps and automatically delivering the samples to the BD COR™ MX instrument for molecular analysis. The BD COR™ MX instrument will perform the analytical steps of the BD SARS-CoV-2/Flu molecular assay, including extraction, amplification and detection.
The system is modular and scalable, and designed to address multiple needs within laboratories for expanding molecular testing and increasing test volumes. The BD COR™ System is particularly well-suited to laboratories requiring high throughput for sample results, minimizing staff interactions and automating labor-intensive and error-prone manual processes.
Caption: The BD COR™ MX/PX System allows 1,700 specimens to be loaded, with onboard capacity for reagents and samples that provide more than six hours of unimpeded system processing and up to 1,000 sample results in 24 hours.
For high resolution please click the image.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 75,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts
BD
Media
Troy Kirkpatrick
VP, Public Relations
+1 858.617.2361
This email address is being protected from spambots. You need JavaScript enabled to view it.
Investors
Francesca DeMartino
SVP, Head of Investor Relations
+1 201.847.5743
This email address is being protected from spambots. You need JavaScript enabled to view it.
Keywords: Humans; COVID-19; Laboratories; SARS-CoV-2; Influenza, Human; Reverse Transcriptase Polymerase Chain Reaction; Diagnostic Tests, Routine; Pandemics; Pathology, Molecular; Public Health; Molecular Diagnostic Techniques; Specimen Handling; Influenza B virus; Influenza A virus; North America
Published by B3C newswire