OEGSTGEEST, The Netherlands, November 30, 2021 / B3C newswire / -- Toxys BV is a biotech company based in Leiden, The Netherlands, that provides innovative, high-fidelity in vitro toxicity screening solutions to identify carcinogenic and other hazardous properties of compounds for the pharmaceutical, chemical, cosmetics and food industry. Today, Toxys announces the creation of its scientific advisory board to help guide the development of innovative animal-free assays to ensure the safety of novel medicines and other products for humans. With the scientific advisory board, Toxys will further accelerate business growth to become an industry-leader in chemical safety assessment.
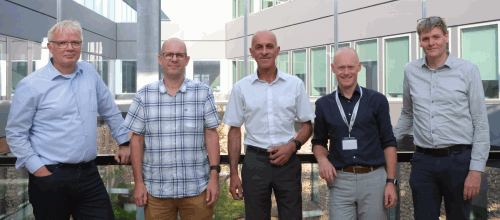
Caption: From left to right: Bob van de Water, Clive Roper, Peter Boogaard, Giel Hendriks, Torben Østerlund.
For high resolution please click the image
“I am extremely excited and proud to be able to announce the appointments of the members of our Scientific Advisory Board. They are all internationally respected scientific experts in the fields of chemical safety assessment and alternatives to animal testing. The expertise from these new scientific board members will further strengthen our R&D activities and is an important step to further enable the growth of Toxys” said Torben Østerlund, CSO of Toxys BV and chair of the SAB.
The members of the new Toxys Board are:
Bob van de Water received his PhD in 1995 from Leiden University. He is a professor in Drug Safety Sciences at the Leiden Academic Centre for Drug Research at Leiden University and head of the Division of Drug Discovery and Safety. He has worked on molecular mechanisms of toxicity for over 30 years. This work led to the development of fluorescent protein reporter platforms to quantify cellular stress responses in relation to chemical and drug exposure and qualify the liability for adverse responses. He is currently the coordinator of the EU-ToxRisk project that worked on integrated testing strategies for read-across, applying new approach methodologies. He also coordinates the recently funded RISK-HUNT3R project that will focus on next generation risk testing strategies.
Clive Roper holds a PhD in in vitro dermal toxicology from Newcastle University. From 1996 to 2021, he worked for Charles River, progressing to Director, in vitro toxicology responsible for skin absorption, regulatory in vitro toxicology, genetox, safety pharmacology, and in vitro respiratory toxicology. Clive has authored and co-authored many posters and abstracts and he is a regular journal peer reviewer. He has developed models resulting in direct replacement of animals in dermal absorption, dermal toxicology and inhalation toxicology. He is a member of the North American 3Rs Collaborative and serves on the microphysiology (MPS) subgroup and a member of the Board of the UK NC3Rs. In 2021, Clive set up his consulting business, Roper Toxicology Consulting Limited, aiding in the development of new approach methodologies with SMEs and supporting regulatory acceptance of products to market.
Peter Boogaard holds degrees in both pharmacy and chemistry, and a PhD in Toxicology from Leiden University. After joining Shell in 1990, he held several positions, including Head of Shell Biomedical Services and Global Discipline Lead & Manager Toxicology with global responsibility for health risk assessments of products and processes. In addition, he is Professor of Environmental Health and Human Biomonitoring at the Division of Toxicology of Wageningen University. He has been the chair of the toxicology group of CONCAWE, member of the scientific council of ECETOC, and other tripartite committees on chemicals risk assessment such as ILSI-HESI and CEFIC’s Long-range Research Programme. He served on several committees of the Health Council of The Netherlands, including the Dutch Expert Committee on Occupational Standards and the Committee for Evaluation of Carcinogenic Substances. In addition, he is a scientific advisor on Risk Assessment for the European Commission and served on the Scientific Committee for Occupational Exposure Limits. He is (co-)author of more than 300 scientific publications and reports, in the fields of occupational toxicology, NAMS, human biomonitoring, dermal exposure, reproductive toxicity, nephrotoxicity and (cancer) risk assessment.
About Toxys
Toxys is a Dutch biotech company that offers a broad spectrum of innovative in vitro toxicology solutions. Toxys was founded in 2014 as a spin-off from the Leiden University Medical Center and has its state-of-the-art laboratory facilities located at the Leiden Bio Science Park. Toxys is expert in toxicological research with a mission to improve animal-free safety testing by creating mechanistic in vitro chemical safety tests to meet the needs for safer medicines, chemicals and cosmetics. Toxys has developed the unique ToxTracker®, ReproTracker® and ToxProfiler™ assays. ToxTracker is a high-throughput stem cell-based reporter suite of assays that allows reliable identification of genotoxic carcinogens. ReproTracker is an animal-free test to identify compounds that are toxic to human embryonic development. ToxProfiler is a human cell assay for mechanistic toxicity testing.
Toxys is currently working with seven of the top ten global Pharma companies and several major chemical, cosmetics and food multinationals and is highly valued for its scientific expertise, high-quality results and responsiveness.
Contact
Giel Hendriks
+31 71 3322474
This email address is being protected from spambots. You need JavaScript enabled to view it.
Keywords: Humans; Toxicity Tests; Chemical Safety; Animal Testing Alternatives; Drug-Related Side Effects and Adverse Reactions; Animals; Carcinogens; Carcinogenesis; Teratogens; DNA Damage; Stem Cells; Biological Assay; Cell Differentiation; Consumer Product Safety; Mutagenicity Tests; Pharmaceutical Preparations; Biotechnology; Industry; Food Industry; Cosmetics; Netherlands
Published by B3C newswire