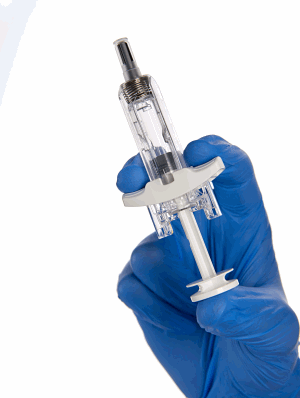
New solution extends BD UltraSafe Plus™ needle guard platform capabilities to deliver up to 2 mL and 30 cP biologics – enhancing self-administration
FRANKLIN LAKES, NJ, USA, November 17, 2021 / B3C newswire / -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the launch of the BD UltraSafe Plus™ 2.25 mL Passive Needle Guard for use by pharmaceutical companies in drug-device combination products. When combined with a Glass Prefillable Syringe, the BD UltraSafe Plus™ 2.25 mL system enables the subcutaneous delivery of biologic solutions of different fill volumes up to 2 mL and viscosities up to 30 cP.
BD UltraSafe Plus™ 2.25 mL is the latest solution to be commercially released into BD’s portfolio of drug delivery systems for combination products and is designed to meet the needs of healthcare providers, patients, and caregivers in performing manual injections of biologic solutions. The unique design of BD UltraSafe Plus™ 2.25 mL complements bio-pharmaceutical companies’ combination product strategies – enabling patient-controlled injection for complex, high-viscosity drugs.
Biologic therapies are often self-administered by patients or caregivers and require delivery systems that provide ease of use and safety benefits for use in non-clinical settings. However, the viscosity and injection volume of biologics has shown a tendency to increase over the last years (>1 mL, >10 cP) that are beyond the capabilities of many commercialized injectable drug delivery systems. Biologic formulations with high volumes and viscosities often require stronger forces to inject, which can create challenges from a user’s perspective.
“With BD UltraSafe Plus™ 2.25 mL, we are innovating drug delivery systems with a goal to provide confidence and ease of use to patients and improve their self-injection experience, and to serve the expanding biologic drug delivery design space,” said Eric Borin, worldwide president, BD Medical – Pharmaceutical Systems (1).
“We are excited to support bio-pharmaceutical drug launches with a new and robust platform technology that is compatible with BD Neopak™ 2.25 mL Glass Prefillable Syringe and supports our customers’ high-speed assembly by leveraging extensive experience with BD UltraSafe™ Passive Needle Guard in commercialized combination products.”
In developing a patient-centric solution, BD UltraSafe Plus™ 2.25 mL was evaluated in a human factors validation study in which usability was demonstrated and the majority of participants expressed confidence that the activated safety mechanism would protect them from needlestick injuries (1).
BD is developing a range of innovative options for bio-pharmaceutical companies seeking to deliver biologics in 2 mL dose volumes, including the BD Neopak™ Glass Prefillable Syringe, BD Neopak™ XtraFlow™ Glass Prefillable Syringe (2), BD Intevia™ Disposable Autoinjector (2), and BD UltraSafe Plus™ Passive Needle Guard.
BD UltraSafe Plus™ 2.25 mL Passive Needle Guard is now available for development by bio-pharmaceutical companies.
For more information on BD UltraSafe Plus™ passive needle guard, please visit: https://drugdeliverysystems.bd.com/products/safety-and-shielding-systems/ultrasafe-plus-passive-needle-guard
For high resolution please click the image.
About BD
BD is one of the largest global medical technology companies and is advancing the world of health by improving medical discovery, diagnostics and care delivery. The company supports the heroes that work on the frontline by developing innovative technology, services and solutions, to advance clinical therapy for patients and clinical process for healthcare providers. BD and its 65,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to healthcare. In 2017, BD welcomed C. R. Bard and its products into the BD family.
Contacts
Trey Hollern
Director, Public Relations
+1 862.284.8629
This email address is being protected from spambots. You need JavaScript enabled to view it.
Nadia Goncalves
Senior Director, Investor Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
+1 201.847.5934
References
1. Human Factors Validation Study - BD UltraSafe Plus™ 2.25 mL passive needle guard (internal study), Le Pont- de-Claix, France; Becton, Dickinson and Company, 2019.
2. BD Intevia™ 2.25 mL disposable autoinjector and BD Neopak™ XtraFlow™ 2.25 mL prefillable syringe are products in development; some statements are forward looking and are subject to a variety of risks and uncertainties.
Keywords: Humans; Injections; Syringes; Self Administration; Health Personnel; Caregivers; Drug Delivery Systems; Subcutaneous Tissue; Viscosity; Biological Products; Biological Therapy; Technology; Pharmaceutical Preparations; North America
Published by B3C newswire